Our products
ENVIRONMENTALLY FRIENDLY DEVICE FOR
PROTECTION AGAINST LIME SCALE AND ALGAE
H2O SYSTEM
Ultrasonic irradiation for the control of blue-green algae with H2O System
Ultrasonic irradiation has recently been evaluated as a rapid alternative for controlling the flowering of algae. The effects of ultrasonic irradiation on blue-green algae were studied, keep in consideration the factors that affect their sedimentation and death.
The natural suspensions of the blue-green algae were treated using an ultrasonic system and the results indicate that this irradiation collapses the gas vacuoles of the blue-green algae and makes it effectively sediment.
Ultrasonic irradiation causes the phenomenon of cavitation. The implosion of the cavitation bubbles produces free radicals, which damage the blue-green algae cells and inflicts immediate damage on the photosynthetic activity. Different tests have shown that once the photosynthetic apparatus in the cell is damaged by ultrasonic irradiation, the activity does not resume and the proliferation of blue-green algae is inhibited.
Further experiments have shown that the ultrasonic irradiation of blue-green algae does not cause the release of toxins into the aquatic environment.
Ultrasonic devices are already applied to ponds, tanks, lakes and tanks to destroy algae and control their development with a low environmental impact technique. Their effectiveness is measured in active radius with respect to the linear distance to which they can irradiate. The algae disappear approximately for a semicircular surface equal to that covered by the active ray.
The term alga, even though it has entered the common use, has in fact little systematic value, with it, we indicates a set of photosynthetic organisms (able to synthesize organic matter, exploiting light as an energy source), unicellular or multicellular inhabitants in places wet, both in fresh water and in marine waters.
Although very many algae are microscopic and measure 1 or 2 micrometers in diameter, in many cases their presence shows up in a flashy form in the form of foam in the ponds, of green encrustations on the trunks of trees or in fountains or in phenomena such as red tides. The macroscopic forms of algae are usually anchored to solid surfaces and grow abundantly in the intertidal and subtidal areas up to depths greater than 250 m, depending on the penetration of light. The algae grow also on the rocks, both in stagnant waters and currents, often detaching from the substrate and floating in the form of foam. Microscopic algae, mainly unicellular and planktonic (freely floating organisms, transported by currents), are an essential part of the food chains of all aquatic habitats.
The algae are subdivided according to the photosynthetic pigments in Rodophytes (red algae), Feofite (brown algae) and Chlorophytes (green algae). Green algae or chlorophytes: green algae are similar to higher plants as they contain both chlorophyll A and chlorophyll B and store the nutrients produced by photosynthesis in the form of starch. Most of them are unicellular or colonial and form an important component of the plankton of freshwater habitats.
USE OF ULTRASOUND AGAINST ALGAL PROLIFERATION
Before and after

Blue-green algae
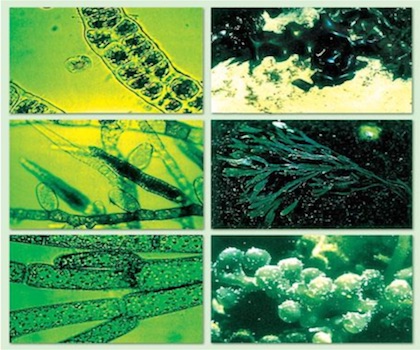
The appearance of algal developments is often caused by changes in the levels and types of factors that can be influenced by human activity.
The main problems caused can be summarized as follows:
- Water can be harmful to health
- The organoleptic characteristics of the water can decline towards non-potability
- Increased vegetation can prevent the smooth flow of water
- Drinking water treatment can become difficult and the water supplied can have an unacceptable taste or odor.
These blooms can die, decompose and produce qualitatively unpleasant water, for example having bad odor, if used as a supply for public or private use, algae can be difficult to remove and therefore can add a questionable taste to the transported water. The algae also have a tendency to absorb and concentrate the mineral substances in their cells. When they die, at the end of the growth period, they settle on the bottom of the watercourses and release them from these substances, thus becoming a form of secondary pollution.
One of the most common symptoms of eutrophication is the development of blue-green algae blooms (Cianobacteria) which can greatly reduce the clarity of water and therefore reduce the amount of light available for phytoplankton. Cyanobacteria are able to live close to the surface of the water by means of special vacuoles filled with gases that allow the plants to float. Once the cyanobacteria blooms or, more generally, of algae reach high concentrations, they can negatively modify the organoleptic characteristics of the water, generating serious problems and interfering with certain treatment processes. When bacterial populations reach very high proportions they can produce toxins that can make water dangerous for consumption.
High levels of nutrients can stimulate other forms of primary production, in addition to algae and cyanobacteria.
The coastal areas of many water bodies enriched with substances are often affected by excessive developments of aquatic macrophytes, which can influence recreational and industrial activities and alter the structure of nutrient photoreceptors. The excessive aquatic development of phytoplankton and macroscopic plants generates aesthetic problems and reduces the value of water in the water body as a recreational resource.
From a purely aesthetic point of view, the clear and crystalline water characteristic of oligotrophic systems is more attractive for swimming and rowing. High phytoplankton concentrations increase water turbidity. The macroscopic plants can completely invade the entire surface making the water almost completely unsuitable for these activities.

DAMAGES CAUSED BY CALCARE
Calcium carbonate is insoluble and therefore precipitates producing scale on plants and exchangers while the carbon dioxide produced generates corrosive effects.The solid limestone layer becomes more and more often over time.
The deposits that are deposited on the walls of the pipes and the resistances create, over time, also very high load losses, and the incrustations on the equipment reduce its efficiency and reliability.
In the civil field in the presence of limestone, the first problems are found in household appliances and sanitary ware: the dishwasher does not work more well, the shower holes get clogged, the washing machine stops, the water in the water heater takes longer to heat up and consumes more energy.
Then you notice that the heating system to maintain the same temperature consumes more. The layer of limestone that has formed in the pipes is insulating and the hot water makes it more difficult to radiate its heat into the house: it is calculated that 1 mm. of calcareous deposit increases consumption by 10-15%. In the upper floors there is always less hot water, the bottlenecks due to limestone decrease the flow until the pipe is completely obstructed.
A clarification: calcium and magnesium deposits are harmful for household appliances, but these salts are not a problem for our health, rather it is demonstrated the importance of the presence of calcium in water for food use, as it prevents cardiovascular disease and strengthen the bones.
Only on medical indications, for those who already suffer from problems of calculations, the use of waters with particular saline grades may be recommended.
Normally the presence of calcium in drinking water is important for health to the point that the law prohibits the lowering of hardness in the case of installation of softeners under 15° F (French degrees).
The incrustations are generally due to the precipitation of poorly soluble or unstable mineral salts, mainly calcium and magnesium bicarbonates which, being unstable compounds even at room temperature, tend to turn into carbonates liberating carbon dioxide according to the scheme:
Ca(HCO3)2 ------> CaCO3 + CO2 + H2O Mg(HCO3)2 ------> MgCO3 + CO2 + H2O
The decomposition of bicarbonates is conditioned by the presence of free CO2 in the water; the amount of CO2 insufficient to prevent it is called equilibrium, the excess quantity is called aggressive; values lower than those of equilibrium determine the precipitation of carbonates even at room temperature.
As the temperature increases, the presence of free CO2 decreases, the bicarbonate decomposition process accelerates and the insoluble calcium and magnesium carbonates precipitate into very adherent deposits with high insulating power.
“From the results of experiments conducted in collaboration with the Department of Public Health Sciences of the University of Modena and Reggio Emilia, it has also emerged that the application of this technology in pulsed mode is able to significantly reduce the formation of biofilm on the walls. of the pipes and to modify their composition, thus offering less chance of survival for the Legionella bacteria ”.
Starting from these experiments we have produced the green model obtaining considerable results in the control of algae and limestone, exploiting the two principles: electromagnetism and ultrasound.
The system actually slows down the formation of calcareous deposits, ferro-calcic and biofilm, extends the life of the systems and components and instruments, can gradually "clean" the plants and gradually restore the project flow rates, improving the overall efficiency of the different systems.
H2O System, applied externally to the water pipes without any invasive work, inhibits the formation of calcareous encrustations and manages to progressively dissolve the pre-existing deposits. The impulsigenerates and subsequently sent to the water direct the precipitation towards a crystalline structure of an acicular shape (Aragonite Trimetrical Group Rhombic System), small white needles with free and not encrusting tips, rather than in the natural form similar to closely agglomerated cubes (Calcite Group Dimetric Trigonal System) which is the one that causes the formation of such hard limestone deposits.
Aragonite

Calcite

The principle of treatment against H2O System is based not on the elimination or disintegration of calcium carbonate, but only and exclusively on the change in the morphology of its crystals.
By modifying the characteristics of the formation environment of calcium carbonate crystals or by forcing the "germination" in a suitable environment in advance, it is possible to cause a structural change in the morphology of the crystals which assume a non-encrusting configuration.
The naturally occurring calcium carbonate crystals have considerable encrusting power, while those that are formed after our treatment do not have such a characteristic, on the contrary they immediately begin to detach the encrustations present.
Our appliances are able to generate the electric balance suitable to favor the formation of non-encrusting calcium carbonate crystals.
The generator works at low voltage, there is no direct electrical contact with the pipeline or even with the liquid, therefore electrolytic phenomena can not be produced; consumption varies according to the models from 1 to 8 V / A and the plant impact is extremely limited, since for the construction of the system there is no need for hydraulic works, flow interruptions, cutting of pipes or insertion of valves and by-pass.
THE ADVANTAGES
DIRECT
- REDUCED WATER CONSUMPTION IN CLOSED CIRCUITS
- BETTER EFFICIENCY IN THERMAL EXCHANGE
- REDUCTION OF ENERGY EXPENSE
- REDUCTION OF THE NUMBER OF MAINTENANCE
- MINOR USE OF CHEMICAL PRODUC
- MINOR DEGRADATION OF THE METAL PARTS
- MINOR DISPOSAL COSTS
INDIRECT
- NO HYDRAULIC WORK
- EXTERNAL APPLICATIONS TO TUBES
- RETURN OF IMAGE FOR RESPECT OF THE ENVIRONMENT
- MINOR STORAGE USE AND DISPOSAL OF PRODUCT
- NO MAINTENANCE WITH CHEMICAL PRODUC
- LOW ENERGY CONSUMPTION
- POSSIBILITY OF POWER SUPPLY WITH SOLAR ENERGIES
External aluminum casing
IP 65 protection rating
Outdoor T° Range -10 -65°C
Room Humidity Range RH
Max T°C of the 80°C pipe
Consumption max 8 W
Power supply 220 V – 50 Hz / 110 V – 60 Hz Working voltage 24 V


ONE limestone protection
for pipes up to 1"

ONE PLUS limestone protection
for pipes up to 2"

TEN limestone protection
for pipes from 3"to 6"

TEN PLUS protection against
limestone and algae for pipes
up to 12" tanks and basins
In the industrial field, the problems caused by calcareous incrustations are present everywhere because all the waters, even the "aggressive" cold ones, become "encrusting" by heating.
The sectors concerned are:






